Abstract
INTRODUCTION: Anaplastic large cell lymphoma, ALK-positive (ALK+ ALCL), is an aggressive CD30 positive T-cell lymphoma. The use of brentuximab vedotin (BV) in the frontline setting has demonstrated improved outcomes. However, data outside of controlled settings such as clinical trials are lacking. Although the overall prognosis of ALK+ ALCL is remarkably better than other T-cell lymphomas, no particular risk factors predictive of early relapse and mortality have been clearly identified. Therefore, we conducted a retrospective review of newly diagnosed ALK+ ALCL managed with systemic therapy and to identify clinico-pathologic characteristics associated with early relapse and mortality.
METHODS: We retrospectively analyzed patients with untreated ALK+ ALCL diagnosed between 2002 and 2020 at The University of Texas MD Anderson Cancer Center. The study outcomes were overall survival (OS) and progression-free survival (PFS). The International Prognostic Index (IPI) and Prognostic Index for T-cell lymphoma (PIT) scores were used for risk stratification. The therapy approaches used during the patients' disease process were divided as follow: BV-based therapy (either alone or in combination with chemotherapy); and non-BV-based therapy. Kaplan-Meier and log-rank test were used for survival analysis. Univariate and multivariate Cox regression analysis were used to estimate hazard ratios (HR) with a 95% confidence interval (CI). Outcomes with a p-value <0.05 were considered statistically significant.
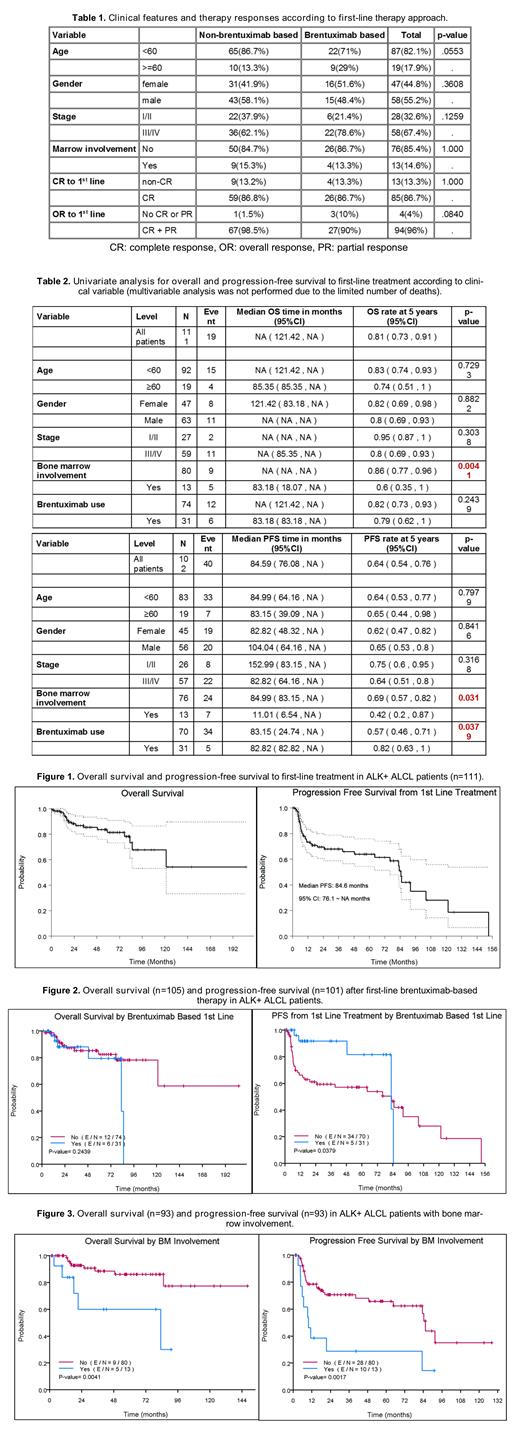
RESULTS: We identified 117 patients, 11 were excluded due to incomplete data. In the remaining 106 patients, the median age at diagnosis was 39 years (range 23-81, 83% <60 years) with a male predominance (57%). Most patients (90%) had ECOG performance status 0-1; 41% had elevated serum lactate dehydrogenase level; 68% had stage III/IV; 15% had bone marrow involvement; 85% had IPI score 0-2 (i.e., low, low-intermediate risk); and 84% had PIT score 0-1 (i.e., low, low-intermediate risk). Table 1 summarizes the clinical features according to the therapy used. BV-based therapy was given to 29% (n=31/106) of patients. Among the 99 patients evaluable for response, overall response (OR) rate to first-line treatment was 96% (95% CI: 90-99%) with complete response (CR) rate of 86.7% (95% CI: 78.6-92.8%). There were no differences in responses between BV and non-BV regimens (Table 1). With a median follow up of 50 months (95% CI: 34.3-65 months), the median OS on the entire cohort was not reached and 4-year OS rate was 85% (95% CI: 78-93%); the median PFS to first-line therapy and 4-year PFS rate were 84.6 months (95% CI: 76.1-not reached) and 66% (95% CI: 56-77%), respectively (Figure 1). In the univariate analysis, frontline BV showed a significant improvement in PFS (5-years 82% versus 57%; p=0.038) but no OS (Table 2, Figure 2). Bone marrow involvement was associated with worse OS (p=0.002) and PFS (p=0.031) (Table 2, Figure 3). Second-line therapy (either with BV or non-BV regimens) was reported in 39 patients; OR rate was 82.1% (95% CI: 66.5-92.5%) with CR rate of 74.4% (95% CI: 57.9-87.0%). However, median PFS (17.5 months) and 4-year PFS rate (41%) were significantly shorter than with first-line therapy. Progression after first- and second-line therapy was seen in 47% and 61% of patients, respectively. Stem cell transplantation was performed in 28% of patients (n=27/95) upon first relapse (autologous n=23; allogeneic n=4 [two upon progression from autologous transplant]); outcomes with this approach will be presented at the meeting.
CONCLUSION: This large cohort of ALK+ ALCL patients was characterized by younger age, good performance status, advanced disease, and low- to intermediate-risk IPI/PIT scores. Although CR rates to first-line therapy were over 85% with both BV- and non-BV-based regimens, only frontline BV-based regimens conferred improved PFS. Furthermore, bone marrow involvement was associated to shorter OS and PFS. Response rates after salvage therapy were high, but with shorter PFS. In conclusion, this study suggest the addition of BV as the preferred regimen in the management of ALK+ ALCL; a more aggressive approach might be considered to those deemed as high risk for early mortality and relapse (i.e., bone marrow involvement). Further analysis with pathologic and molecular studies are ongoing and results will be reported at the meeting.
Jain: Lilly: Membership on an entity's Board of Directors or advisory committees; Kite: Consultancy. Chihara: Astrazeneca: Honoraria. Steiner: BMS: Research Funding; Seattle Genetics: Research Funding; Rafael Pharmaceuticals: Research Funding. Samaniego: Arog: Research Funding; Imbrium: Membership on an entity's Board of Directors or advisory committees. Ahmed: Tessa Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Seagen: Research Funding; Xencor: Research Funding; Merck: Research Funding. Fowler: Bristol Myers Squibb, F. Hoffmann-La Roche Ltd, TG Therapeutics and Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; BostonGene, Corp: Current Employment, Current holder of stock options in a privately-held company. Nastoupil: Takeda: Honoraria, Other: DSMC, Research Funding; Caribou Biosciences: Research Funding; Janssen: Honoraria, Research Funding; Gilead/Kite: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; MorphoSys: Honoraria; TG Therapeutics: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Bayer: Honoraria; Epizyme: Honoraria, Research Funding; ADC Therapeutics: Honoraria; Bristol Myers Squibb/Celgene: Honoraria, Research Funding; IGM Biosciences: Research Funding; Denovo Pharma: Other: DSMC. Hosing: Nkarta Therapeutics: Membership on an entity's Board of Directors or advisory committees. Vega: CRISPR Therapeutics and Geron: Research Funding; i3Health, Elsevier, America Registry of Pathology, Congressionally Directed Medical Research Program, and the Society of Hematology Oncology: Research Funding. Pinnix: Merck Inc: Research Funding. Wang: OMI: Honoraria; VelosBio: Consultancy, Research Funding; The First Afflicted Hospital of Zhejiang University: Honoraria; CAHON: Honoraria; BioInvent: Research Funding; Dava Oncology: Honoraria; CStone: Consultancy; Kite Pharma: Consultancy, Honoraria, Research Funding; InnoCare: Consultancy, Research Funding; Imedex: Honoraria; Lilly: Research Funding; BeiGene: Consultancy, Honoraria, Research Funding; Moffit Cancer Center: Honoraria; Epizyme: Consultancy, Honoraria; Hebei Cancer Prevention Federation: Honoraria; Clinical Care Options: Honoraria; Miltenyi Biomedicine GmbH: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding; Newbridge Pharmaceuticals: Honoraria; Genentech: Consultancy; Chinese Medical Association: Honoraria; Molecular Templates: Research Funding; DTRM Biopharma (Cayman) Limited: Consultancy; BGICS: Honoraria; Celgene: Research Funding; Bayer Healthcare: Consultancy; Mumbai Hematology Group: Honoraria; Physicians Education Resources (PER): Honoraria; Scripps: Honoraria; Pharmacyclics: Consultancy, Research Funding; Juno: Consultancy, Research Funding; Oncternal: Consultancy, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; Loxo Oncology: Consultancy, Research Funding; Anticancer Association: Honoraria; Acerta Pharma: Consultancy, Honoraria, Research Funding. Neelapu: Takeda Pharmaceuticals and related to cell therapy: Patents & Royalties; Kite, a Gilead Company, Merck, Bristol Myers Squibb, Novartis, Celgene, Pfizer, Allogene, Kuur, Incyte, Precision BioSciences, Legend, Adicet Bio, Calibr, and Unum Therapeutics: Other: personal fees; Kite, a Gilead Company, Bristol Myers Squibb, Merck, Poseida, Cellectis, Celgene, Karus Therapeutics, Unum Therapeutics (Cogent Biosciences), Allogene, Precision BioSciences, Acerta and Adicet Bio: Research Funding; Kite, a Gilead Company, Merck, Bristol Myers Squibb, Novartis, Celgene, Pfizer, Allogene Therapeutics, Cell Medica/Kuur, Incyte, Precision Biosciences, Legend Biotech, Adicet Bio, Calibr, Unum Therapeutics and Bluebird Bio: Honoraria. Flowers: Kite: Research Funding; BeiGene: Consultancy; EMD: Research Funding; Janssen: Research Funding; AbbVie: Consultancy, Research Funding; Xencor: Research Funding; Cancer Prevention and Research Institute of Texas: CPRIT Scholar in Cancer Research: Research Funding; Burroughs Wellcome Fund: Research Funding; Eastern Cooperative Oncology Group: Research Funding; Celgene: Consultancy, Research Funding; Denovo: Consultancy; TG Therapeutics: Research Funding; Biopharma: Consultancy; Takeda: Research Funding; Ziopharm: Research Funding; Sanofi: Research Funding; Genentech/Roche: Consultancy, Research Funding; Genmab: Consultancy; Gilead: Consultancy, Research Funding; Epizyme, Inc.: Consultancy; National Cancer Institute: Research Funding; Karyopharm: Consultancy; Pharmacyclics/Janssen: Consultancy; SeaGen: Consultancy; Bayer: Consultancy, Research Funding; Guardant: Research Funding; Spectrum: Consultancy; Morphosys: Research Funding; Novartis: Research Funding; Pfizer: Research Funding; 4D: Research Funding; Iovance: Research Funding; Nektar: Research Funding; Cellectis: Research Funding; Acerta: Research Funding; Amgen: Research Funding; Allogene: Research Funding; Adaptimmune: Research Funding; Pharmacyclics: Research Funding. Iyer: CRISPRX: Research Funding; Seattle Genetics: Research Funding; Rhizen: Research Funding; Merck: Research Funding; Legend: Research Funding; Innate: Research Funding; Spectrum: Research Funding; Trillium: Research Funding; Astra Zeneca: Research Funding; Yingli: Research Funding; Cyclacel: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal